Blog
Radiomic Features (Shape, Texture, Intensity) for Prostate Lesion Classification
Radiomics is the process of converting medical images, such as MRIs, into a vast amount of high-dimensional, quantitative data. This powerful technique allows us to see beyond what the human eye can perceive, unlocking hidden patterns within the images. By extracting specific radiomic features—like shape, texture, and intensity—from a prostate MRI, we can develop powerful biomarkers. These biomarkers are crucial for differentiating between benign and malignant prostate lesions, offering a more objective and data-driven approach to diagnosis. This method complements the expert eye of a radiologist, adding a layer of computational precision to improve diagnostic confidence and accuracy.
What Are Radiomic Features and Why They Matter
While a radiologist’s trained eye is indispensable for interpreting medical scans, radiomics introduces a new dimension to this process. It bridges the gap between qualitative visual assessment and quantitative, data-driven analysis. This shift not only enhances diagnostic capabilities but also lays the groundwork for powerful AI-driven tools that can standardize and improve patient care.
The concept of radiomics in medical imaging
At its core, radiomics is a method that extracts a large number of quantitative features from medical images. Instead of just looking at an image and describing what is seen, radiomics uses computational algorithms to measure specific characteristics of the tissue shown in the scan. Think of it as giving a medical image a detailed “fingerprint.” For a prostate MRI, this means analyzing every part of a suspicious lesion to capture its unique properties as numerical data.
These quantitative descriptors go far beyond simple measurements like size. They can describe the complexity of a lesion’s shape, the subtle variations in its brightness, and the intricate patterns of its internal structure. By converting these visual details into standardized data, we create a rich, mineable dataset that can reveal correlations between image patterns and the underlying biology of the tissue, such as its likelihood of being cancerous.
From visual interpretation to quantitative analysis
Traditionally, radiologists interpret prostate MRIs by visually assessing features and assigning a score, like the PI-RADS score. This system, while effective, contains a degree of subjectivity. Different experts might interpret the same subtle visual cues in slightly different ways, leading to inter-reader variability. Radiomics offers a solution by providing objective, repeatable measurements that supplement the radiologist’s judgment.
By quantifying a lesion’s characteristics, radiomic features prostate MRI analysis provides a stable, mathematical foundation for diagnosis. It’s not about replacing the radiologist but empowering them with an additional layer of objective evidence. This fusion of human expertise and computational data leads to more confident and consistent prostate cancer lesion classification. The use of quantitative imaging biomarkers derived from radiomics helps standardize the diagnostic process, ensuring that assessments are less dependent on individual interpretation and more rooted in reproducible data. This transition from a purely visual field to one augmented by quantitative analysis marks a significant step forward in precision medicine.
Types of Radiomic Features in Prostate MRI
Radiomic features can be categorized into three main groups: those describing shape, those describing signal intensity, and those describing texture. Each category provides a unique set of information about a prostate lesion, and together they create a comprehensive profile that helps in determining its nature.
Shape-based features
Shape-based features describe the morphology, or physical form, of a lesion. These metrics provide insights into how a tumor is growing and interacting with the surrounding tissue. Malignant tumors often exhibit uncontrolled, infiltrative growth, which results in more irregular and complex shapes compared to benign lesions that tend to be more uniform and well-defined.
Key shape-based features include:
- Volume and Surface Area: These are fundamental measurements of a lesion’s size. While larger lesions are often more concerning, these metrics are most powerful when used in combination with other features.
- Compactness and Sphericity: These features measure how close a lesion’s shape is to a perfect sphere. A high sphericity value indicates a round, regular shape, which is often associated with benign growths. In contrast, cancerous lesions may have lower sphericity and compactness, reflecting an irregular, sprawling structure.
- Elongation and Flatness: These metrics describe the lesion’s proportions, indicating whether it is stretched out in one or two directions. An elongated or flat shape could suggest that the tumor is growing along a specific anatomical plane or structure.
By quantifying these morphological characteristics, shape-based features help translate the visual impression of a lesion’s regularity into objective data that can be used to assess its potential for malignancy.
First-order intensity features
First-order intensity features, also known as histogram-based statistics, describe the distribution of voxel intensities within a lesion without considering their spatial relationship. In simple terms, they analyze the brightness levels of the pixels that make up the lesion on an MRI scan. These features provide a snapshot of the overall signal characteristics of the tissue.
Common first-order features include:
- Mean, Median, and Standard Deviation: These statistics describe the average intensity of the lesion, the central intensity value, and the degree of variation in intensity across the lesion.
- Skewness: This measures the asymmetry of the intensity distribution. A positive skew might indicate a tail of very bright pixels, while a negative skew could suggest a tail of dark pixels.
- Kurtosis: This describes the “peakedness” of the intensity distribution. A high kurtosis value suggests that the intensity values are concentrated around a single peak with long tails, which can indicate unusual tissue properties.
- Energy and Entropy: Energy measures the uniformity of the intensity distribution, with higher values indicating a more homogenous lesion. Entropy measures the randomness or unpredictability of the intensities, with higher values pointing to more heterogeneity.
These intensity metrics are valuable because different tissue types—healthy, inflamed, or cancerous—often have distinct signal intensities on MRI sequences.
Texture features
Texture features are among the most powerful radiomic descriptors because they capture the spatial arrangement of voxel intensities. They measure intra-lesion heterogeneity, which is a key hallmark of cancer. Tumors are often a chaotic mix of different cell types, necrosis, and blood vessels, creating a complex and heterogeneous texture that is not present in healthy tissue.
Texture analysis goes beyond just looking at brightness levels; it examines the patterns and relationships between neighboring pixels. Key texture analysis methods include:
- Gray-Level Co-occurrence Matrix (GLCM): This method analyzes how often different combinations of pixel brightness values occur in a given spatial relationship. It can measure properties like contrast, correlation, and homogeneity within the lesion.
- Gray-Level Run-Length Matrix (GLRLM): This method identifies consecutive pixels with the same intensity value along specific directions. It is useful for detecting coarse or fine textures within the tissue.
- Neighborhood Gray-Tone Difference Matrix (NGTDM): This method quantifies the difference between a pixel’s intensity and the average intensity of its neighbors, providing insights into the coarseness of the texture.
These advanced metrics allow us to quantify the subtle, almost imperceptible patterns that signify tumor heterogeneity.
Radiomic Feature Extraction and Processing
Extracting meaningful radiomic features is a multi-step process that requires careful preparation and computational analysis. Before any features can be calculated, the MRI images must be standardized and the lesion of interest accurately identified. From there, sophisticated software tools and statistical methods are used to extract, select, and refine the features for use in clinical models.
Image pre-processing and segmentation
The first step in any radiomics workflow is preparing the images. This is crucial for ensuring that the extracted features are reliable and comparable across different patients and scanners. Pre-processing steps often include:
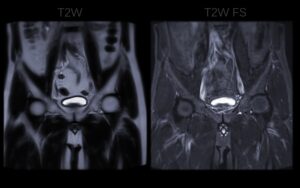
- Normalization: Adjusting the intensity values of the MRI scans to a standard range. This corrects for variations in signal intensity caused by different scanner settings or patient physiology.
- Resampling: Ensuring that all images have the same voxel size and orientation. This standardizes the spatial resolution of the images so that features like shape and texture are calculated consistently.
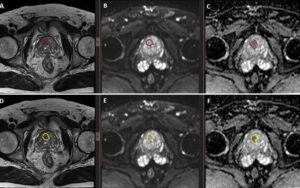
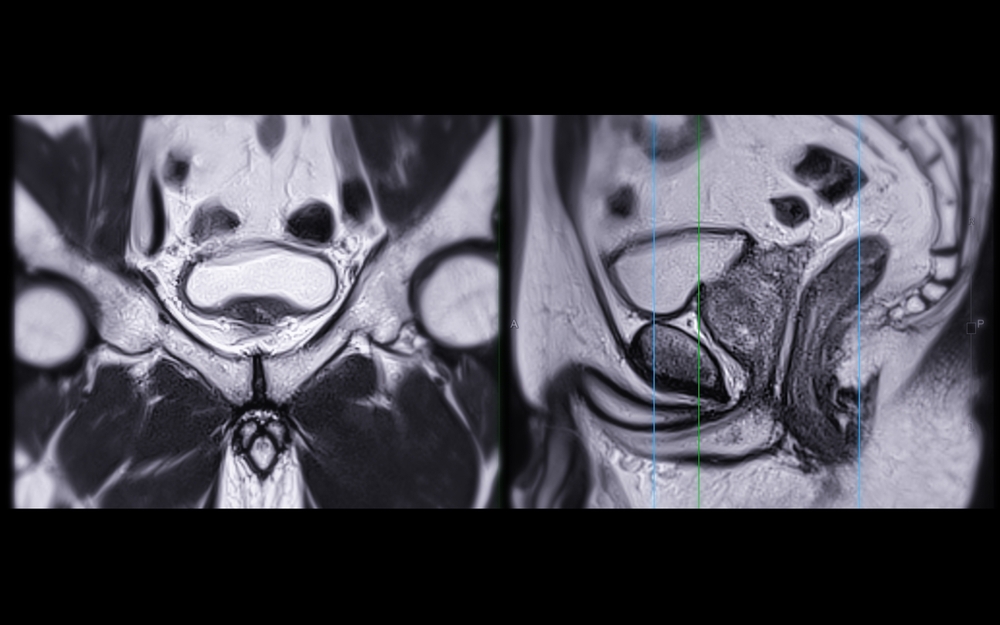
After pre-processing, the next critical step is segmentation. This involves accurately delineating the boundary of the prostate lesion on the MRI scans, typically on a slice-by-slice basis to create a 3D volume of interest (VOI). Segmentation can be done manually by a radiologist, semi-automatically with software assistance, or fully automatically using advanced AI algorithms. The quality of the segmentation is paramount, as an inaccurate boundary will lead to the extraction of flawed and unreliable radiomic features.
Software tools and standardization
Once the lesion is segmented, specialized software is used to automatically calculate hundreds or even thousands of radiomic features from the VOI. One of the most widely used open-source tools for this purpose is PyRadiomics, a Python package that allows for standardized and reproducible feature extraction from medical images.
To ensure that features are calculated consistently across different studies and software platforms, the Image Biomarker Standardisation Initiative (IBSI) was established. IBSI provides standardized definitions and reference values for many radiomic features, promoting reproducibility in research. Adhering to standards like those from IBSI is essential for building trust in radiomics and paving the way for its clinical adoption. These tools and standards help ensure that a feature like “sphericity” is measured the same way in a hospital in one city as it is in a research center in another.
Feature selection and dimensionality reduction
The extraction process often generates a very large number of features—sometimes thousands. Many of these features may be redundant (highly correlated with each other) or irrelevant to the clinical question at hand (e.g., distinguishing benign from malignant lesions). Using all of these features to train a predictive model can lead to overfitting, where the model performs well on the training data but fails to generalize to new, unseen data.
To address this, feature selection and dimensionality reduction techniques are applied. These methods aim to identify the most informative and robust subset of features. This “weeding out” process helps create simpler, more stable, and more interpretable models. By focusing only on the features that provide the most predictive power, we can build AI and machine learning tools that are both accurate and reliable.
Clinical and Research Applications of Radiomic Features
The true value of radiomic features lies in their ability to translate image data into clinically actionable insights. In the context of prostate cancer, these features are being used to improve diagnosis, guide treatment decisions, and predict patient outcomes. They are powerful noninvasive biomarkers that offer a deeper understanding of a tumor’s characteristics.
Lesion classification and risk stratification
One of the primary applications of radiomics is in lesion classification—differentiating benign prostate conditions, like hyperplasia or prostatitis, from malignant cancer. Machine learning models trained on radiomic features have shown great promise in accurately identifying cancerous lesions. These models can learn the subtle patterns of shape, intensity, and texture that distinguish different tissue types.
Furthermore, radiomics can help in risk stratification. For patients diagnosed with prostate cancer, radiomic features have been shown to correlate with the Gleason score, a key indicator of tumor aggressiveness. A model might learn, for example, that a highly irregular shape combined with a heterogeneous texture is strongly associated with a high Gleason score. This information can help clinicians determine the most appropriate course of action, whether it be active surveillance for low-risk disease or immediate intervention for more aggressive cancer.
Radiomics as imaging biomarkers
Radiomic features serve as powerful imaging biomarkers. Unlike traditional biomarkers that may require an invasive biopsy, radiomic biomarkers are derived noninvasively from standard MRI scans. They provide a quantitative snapshot of the tumor’s phenotype—its observable characteristics—which reflects its underlying biology.
For instance, texture features that capture intra-lesion heterogeneity can act as a surrogate for tumor aggressiveness. A more heterogeneous tumor is often more aggressive and may be more resistant to certain treatments. By using these features as biomarkers, clinicians can gain a deeper understanding of a patient’s specific disease without the need for additional invasive procedures. This opens the door to personalized medicine, where treatment strategies can be tailored based on the unique characteristics of a patient’s tumor as seen on an MRI.
Radiomics and machine learning integration
Radiomics and machine learning are a natural fit. Machine learning models, such as Support Vector Machines (SVM), random forests, and deep learning neural networks, are exceptionally good at finding complex patterns in large datasets. The thousands of features generated by a radiomics analysis provide the perfect input for these algorithms.
A typical workflow involves training a model on a large dataset of prostate MRIs where the final diagnosis (from a biopsy) is known. The model learns to associate specific combinations of radiomic features with outcomes like the presence of cancer or a high Gleason score. Once trained, this model can then be used to predict the outcome for new patients. This integration of radiomics and machine learning is the foundation of many modern AI tools for medical imaging.
Challenges and Limitations
While radiomics holds immense potential, several challenges must be addressed to ensure its widespread and reliable clinical application. These challenges primarily revolve around variability in data acquisition and the need for rigorous validation to ensure that models are both reproducible and generalizable.
Variability in acquisition and segmentation
The values of radiomic features can be sensitive to variations in how MRI scans are acquired. Different MRI scanners, manufacturers, and imaging protocols can all introduce slight differences in the resulting images, which can in turn affect the calculated feature values. For example, a texture feature might appear different if the image resolution or noise level changes.
Similarly, variability in lesion segmentation can have a significant impact. Even with expert radiologists, there can be minor differences in how they delineate a lesion’s boundary. These small variations can alter the calculation of shape and texture features, potentially affecting the final output of a radiomic model. Achieving consistency in both image acquisition and segmentation is a critical challenge for the field.
Reproducibility and harmonization across scanners
For radiomics to be a reliable clinical tool, its results must be reproducible. A radiomic signature identified at one hospital should be identifiable using data from another hospital’s scanner. This requires a concerted effort toward harmonization—the process of standardizing imaging and analysis protocols to minimize technical variability.
Initiatives are underway to develop methods that can correct for scanner-specific effects, making radiomic features more robust and comparable across different sites. This is a crucial step for conducting large-scale, multi-center studies and for deploying radiomic-based AI tools in diverse clinical environments.
Overfitting and lack of generalizability
A major risk in developing radiomic models is overfitting. This occurs when a model learns the “noise” or random fluctuations in the training data too well, instead of the underlying patterns. An overfit model may perform exceptionally well on the data it was trained on but fail to generalize to new, independent datasets.
To avoid this, it is essential to perform rigorous validation. This involves testing the model on external datasets from different institutions and patient populations. This process helps ensure that the model has learned true biological signals and is not just memorizing the idiosyncrasies of the training data. Careful validation is the only way to build trust in a model’s predictive power and ensure it will perform reliably in a real-world clinical setting.
Future Directions in Radiomics
The field of radiomics is rapidly evolving, with exciting new research directions pointing toward more powerful and integrated diagnostic models. The future lies in combining radiomics with other advanced technologies and data sources to create a holistic view of a patient’s disease.
Deep radiomics and hybrid models
Traditional radiomics relies on “handcrafted” features that are designed based on our current understanding of image characteristics. A new approach, often called deep radiomics, uses deep learning models, particularly convolutional neural networks (CNNs), to automatically learn the most relevant features directly from the images. These models can identify complex, high-level patterns that may not be captured by handcrafted features.
Hybrid models that combine the strengths of both approaches are also emerging. These models might use deep learning to extract a rich set of features and then combine them with carefully selected handcrafted features and clinical data to make a final prediction. This fusion of automated feature discovery and expert-driven feature engineering holds the potential to create even more accurate and robust diagnostic tools.
Combining radiomics with genomics and clinical data
The concept of radiogenomics aims to find correlations between radiomic features (the tumor’s appearance on an image) and its genomic profile (its genetic makeup). By linking imaging data with genomic data, researchers hope to develop noninvasive methods for predicting a tumor’s genetic mutations, molecular pathways, and potential response to targeted therapies.
Future predictive models will likely integrate data from multiple sources. An AI system might combine a patient’s radiomic features, genomic data, blood test results (like PSA levels), and clinical history to generate a comprehensive risk score or treatment recommendation. These integrated prediction models represent a major step toward a truly personalized approach to cancer care.
Standardization and open databases
To accelerate progress in the field, there is a growing movement toward creating large, standardized, and publicly available databases for prostate MRI radiomics. These open databases and benchmarking initiatives allow researchers from around the world to test and compare their algorithms on a common set of data.
Shared datasets promote collaboration, help establish performance benchmarks for new models, and facilitate the development of more generalizable AI tools. By working together to standardize data and methodologies, the research community can more effectively overcome the challenges of reproducibility and validation, bringing the promise of radiomics closer to routine clinical practice.
Conclusion
Radiomic features—shape, texture, and intensity—are transforming medical imaging from a qualitative art into a quantitative science. By converting prostate MRI scans into rich, actionable data, these features provide objective and reproducible biomarkers that enhance diagnostic accuracy. They help differentiate benign from malignant lesions, assess tumor aggressiveness, and provide a deeper understanding of a tumor’s underlying biology, all noninvasively. Radiomics serves as the crucial bridge between medical imaging and the power of artificial intelligence, paving the way for a new generation of data-driven tools that support clinicians and improve patient outcomes in the fight against prostate cancer.
Pioneering Cancer Detection with AI and MRI (and CT)
At Bot Image™ AI, we’re on a mission to revolutionize medical imaging through cutting-edge artificial intelligence technology.
Contact Us