“Without a doubt, every urologist dealing with prostate conditions should incorporate ProstatID into their practice. Its indispensable role in improving diagnostic accuracy and guiding therapeutic interventions makes it an indispensable tool in the realm of urology. To forego the utilization of ProstatID would be a disservice to both patients and practitioners alike.”
“We are on board with ProstatID. I feel more comfortable with this AI backing me up.”
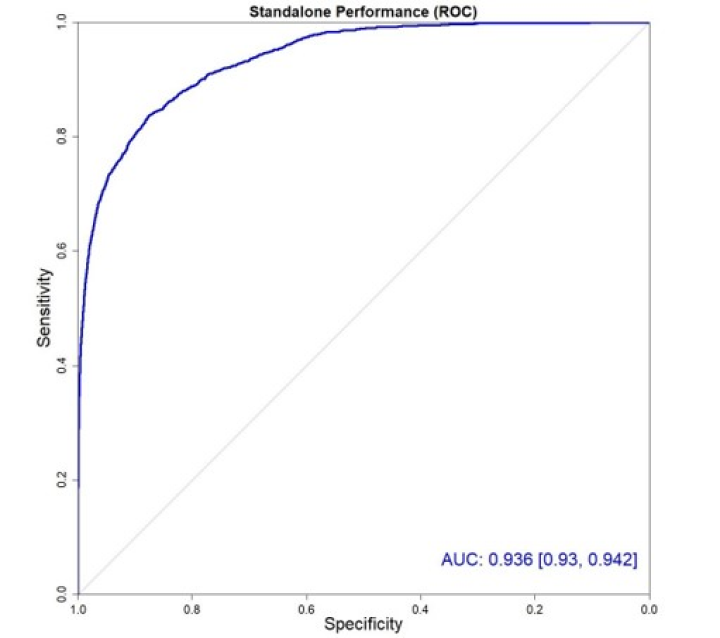
“I have come to rely upon the ProstatID output to serve as my own Quality control of interpretation, and it has paid off.”
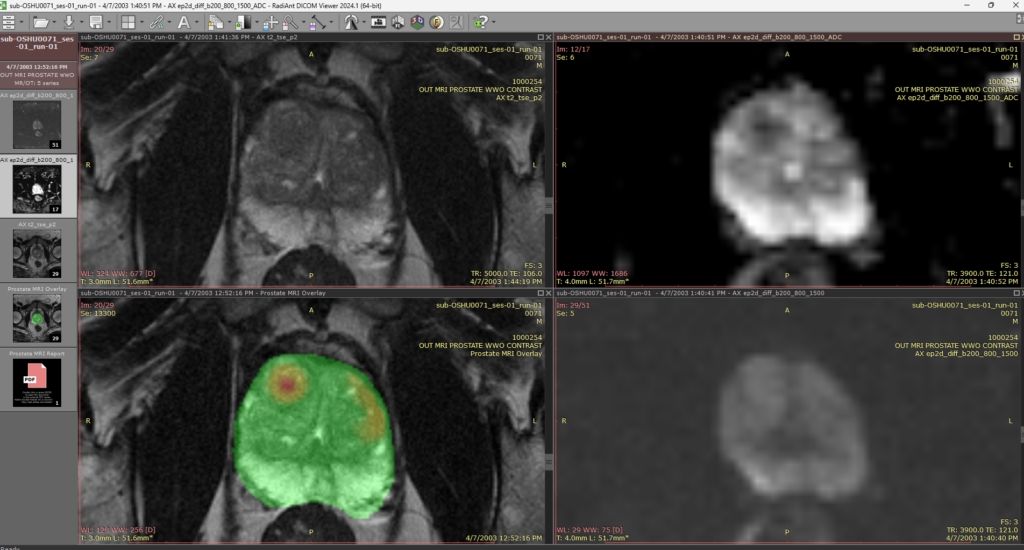
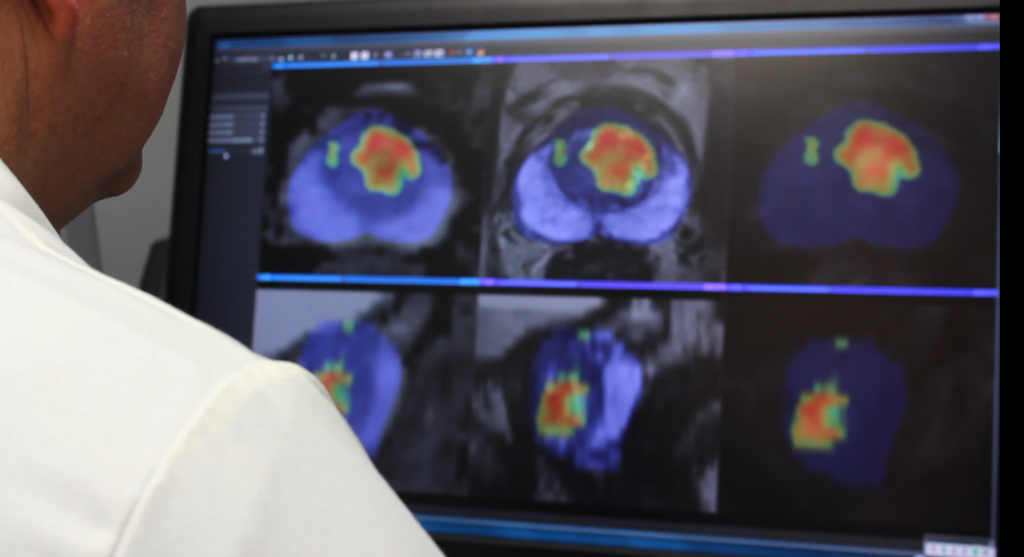
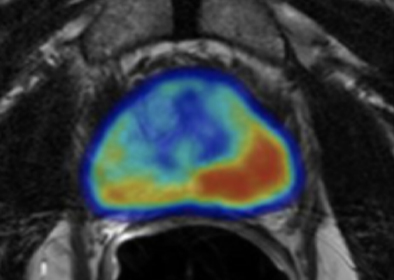
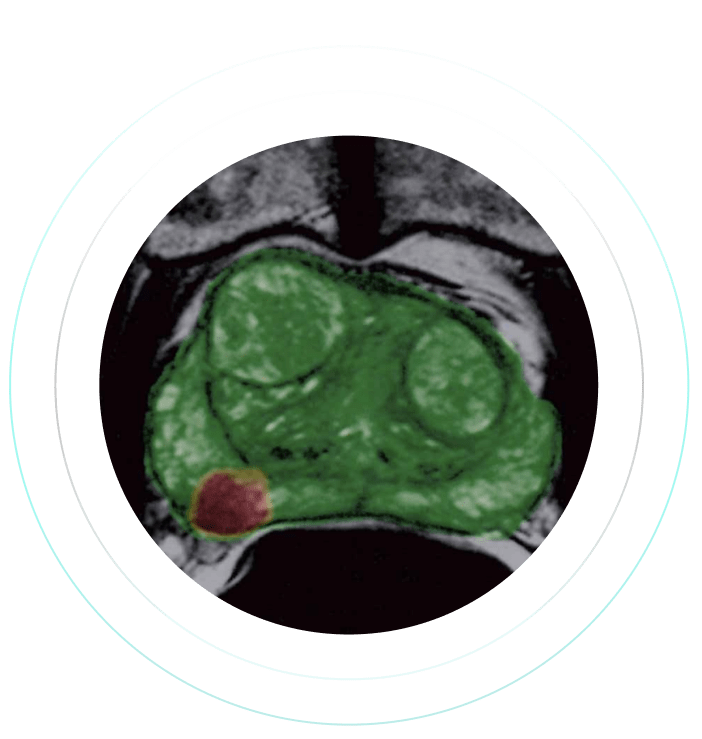
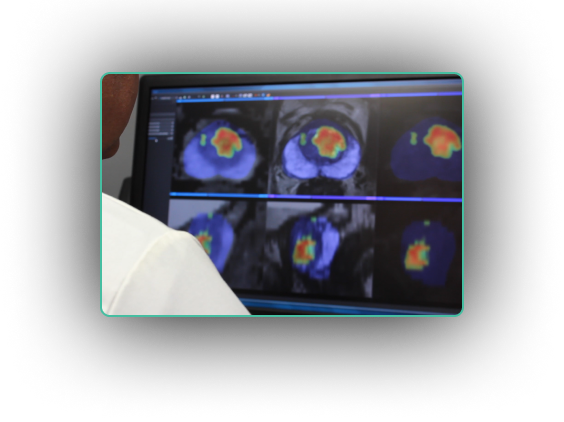
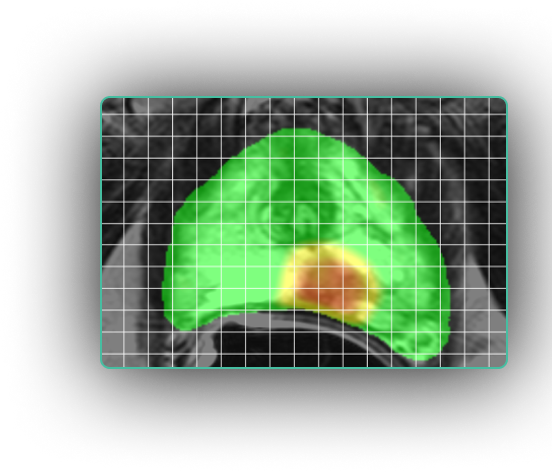
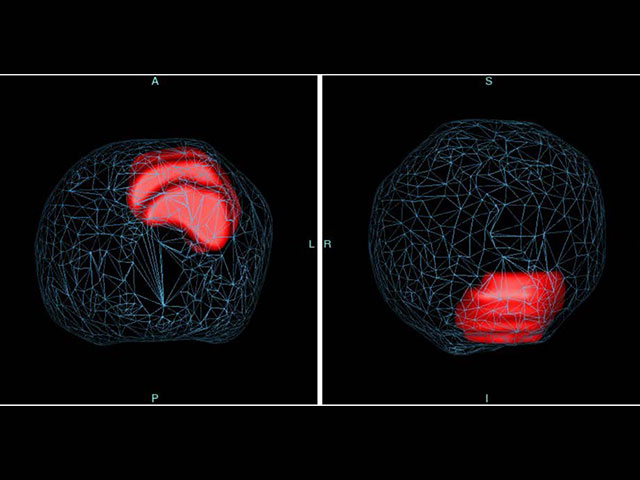
“I use ProstatID on every case. I first look at all of the colorized suspect lesions,
then study them and make my diagnosis. Usually, I follow ProstatID 100%.
ProstatID has been right-on with biopsy confirmation.”
“I’ve known Randy as a friend, a patient-advocate, and, yes-as a nerdy physicist. His ingenuity and eye for the future are only matched by his unflagging support for the health of humanity. This Software gives us a glimpse of how empathy and technology can collide for the good of us all.”
“I have the good fortune of knowing Randy (Randall W.) Jones for over twenty years, through our shared interest in optimizing MRI for the care of men with prostate cancer. Over all these years, Randy has deeply impressed me with his unflagging enthusiasm, innovation, and knowledge. His passion for advancing medical care continues to drive him forward as he pushes the envelope of imaging technology, building on his unique blend of training, experience, and entrepreneurship.”
“In this wonderful book, Randy Jones articulates a story of success. It’s a triumph of creativity, science, and entrepreneurship. This prostate cancer survivor is a nonconformist that responds to challenges with inspiring thought, candor, and conviction. Through artificial intelligence, Jones has been able to identify lesions in a way the world has yet to see and I’m confident th medical field will endorse. Ag great educational read from a brave American spirit.”
“The problem with most translational science is that you seldom get good feedback between the scientist and the end user. By being relentless, actively working on both ends of the process without the middlemen, and learning from failures, he has achieved what no one else can. Physicians are trained to understand a complex system and work within it. Randy reimagines the system itself. The Elon Musk of modern MRI development.”